Real-World Data (RWD) Statistical Analysis Support
-
0 sales
- 23 views
- Save
0 /5.0
User reviewClinical and Observational Data Analysis for Real-World Evidence (RWE) Generation
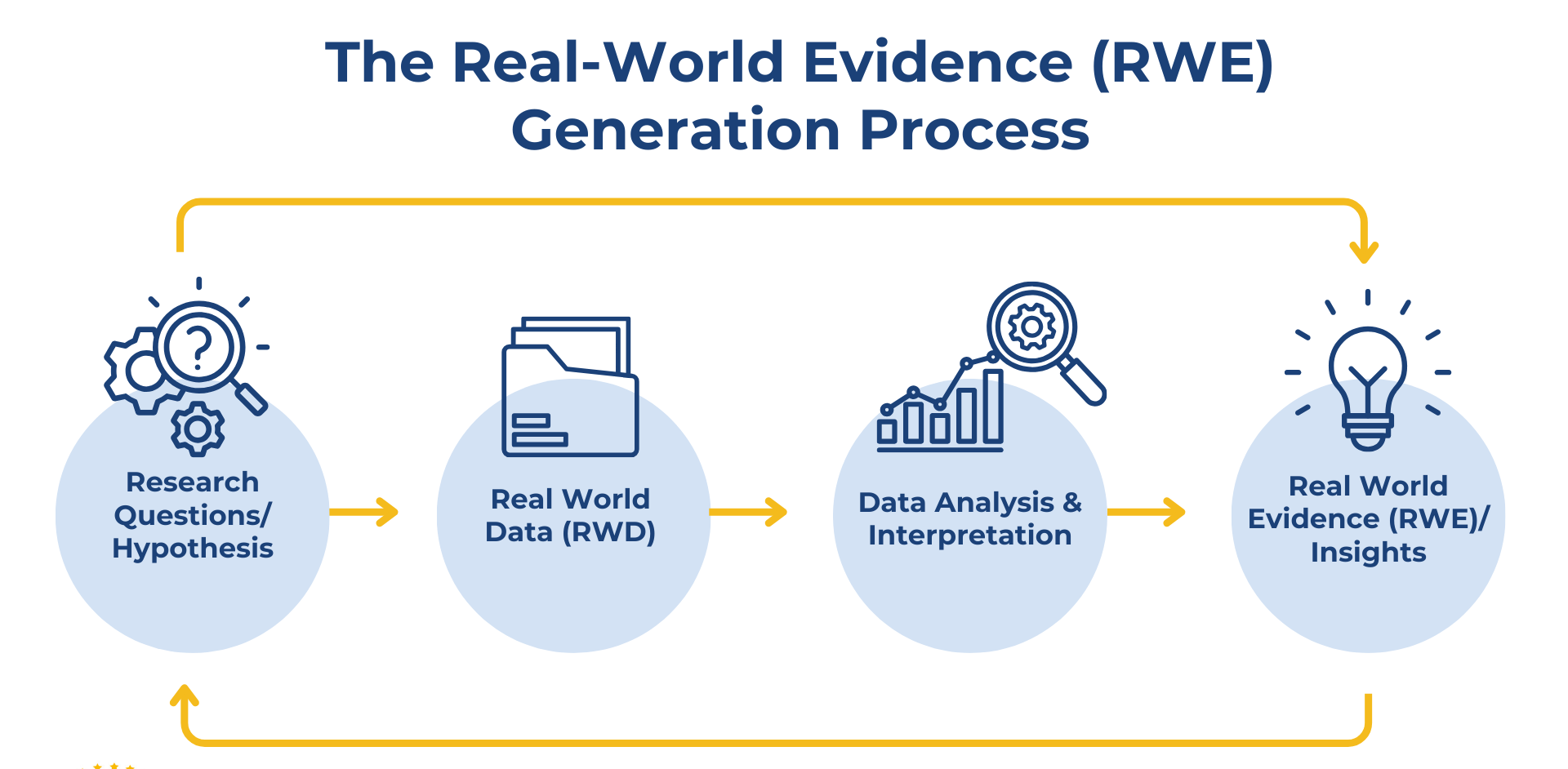
Are you working with registry, claims, or electronic health record (EHR) data to generate real-world evidence? This service provides end-to-end statistical support for the design, analysis, and interpretation of real-world datasets in compliance with STROBE and regulatory RWE standards.
From data preparation to publication-ready results, I help clinical researchers, pharmaceutical companies, and academic teams transform raw RWD into robust, reproducible insights suitable for peer-reviewed journals and health technology assessments (HTA).
What’s Included
- Study design consultation (retrospective, prospective, or hybrid RWD studies)
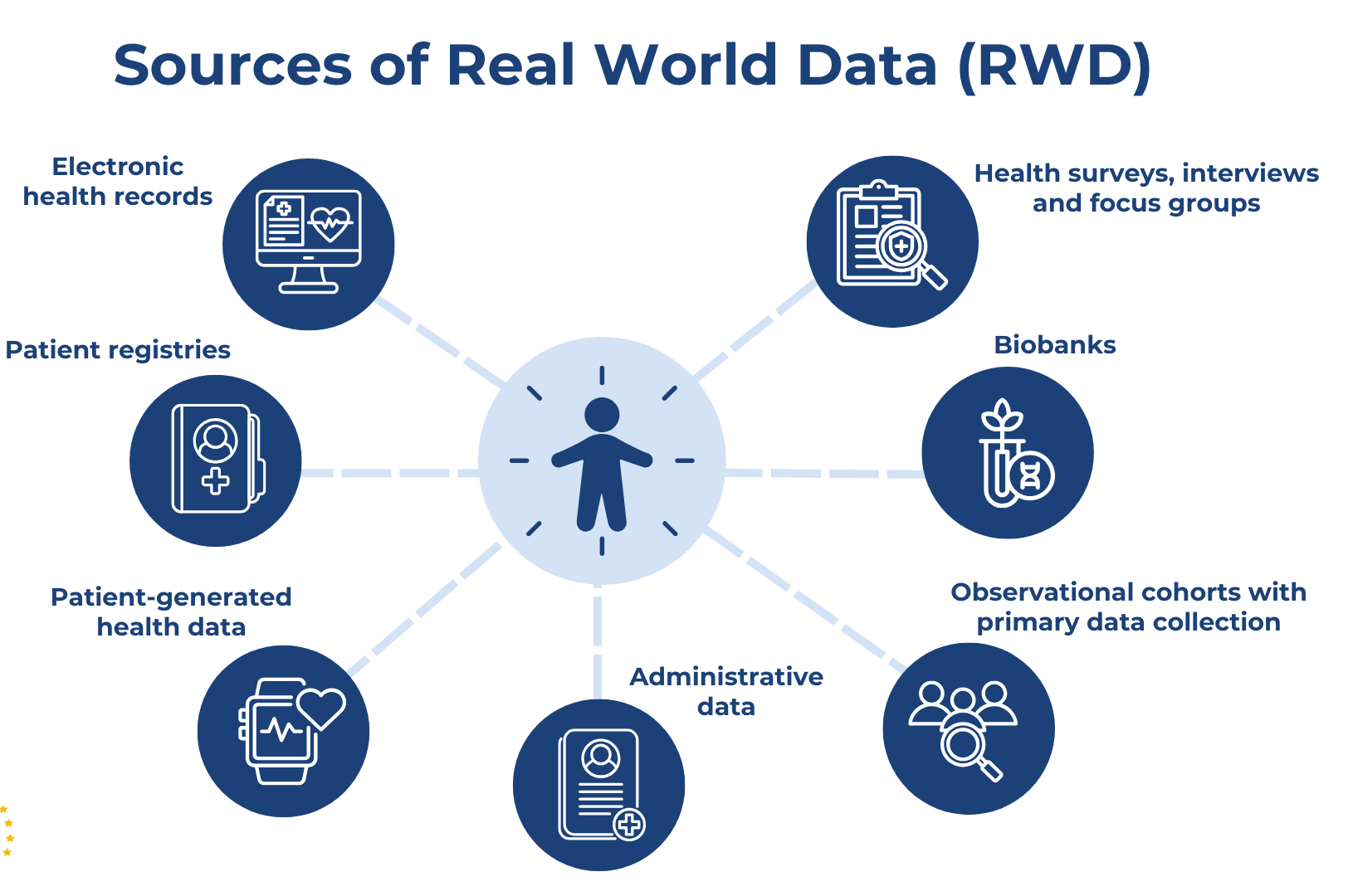
- Data extraction, cleaning, and transformation from registries or EHRs
- Descriptive statistics and baseline cohort profiling
- Comparative effectiveness and outcome analyses
- Logistic, linear, Poisson, and Cox regression models
- Time-to-event and competing risk survival analyses
- Propensity score matching, weighting, and adjustment
- Handling of missing data using multiple imputation
- Subgroup, sensitivity, and interaction analyses
- Generation of Tables, Figures, and Listings (TFLs) for publication
- Syntax/code (R, SAS, SPSS, or Stata) for full transparency and reproducibility
Expertise
- Data Types: Electronic health records (EHR), registry data, insurance claims, hospital discharge datasets, device registries, and observational cohorts
- Software: R, SAS, SPSS, Stata
- Methodological Frameworks: STROBE, ISPE RWE guidelines, FDA & EMA RWE frameworks
- Publication Standards: APA 7th, AMA, CONSORT, and ICH E9(R1) reporting compliance
- Confidentiality: Secure data handling and NDA available upon request
Fequently asked questions
1. What types of cohort studies do you support?
I work with both prospective and retrospective cohort studies, including registry-based, hospital-based, and population-based datasets, as well as real-world evidence studies.
2. How do you ensure patient data privacy?
All data are handled under strict confidentiality, stored securely, and never shared with third parties. I can sign an NDA or data use agreement if required.
3. Which statistical methods are typically used for cohort analysis?
I apply logistic regression, Poisson regression, Cox proportional hazards, Fine–Gray competing risks, and longitudinal mixed models depending on your study design and outcomes.
4. Can you analyse time-to-event outcomes in cohort studies?
Yes, I conduct survival analysis using Kaplan–Meier, Cox models, and competing risk methods for outcomes such as mortality, disease progression, or treatment response.