Retrospective Study Statistical Analysis Support
-
1 sales
- 19 views
- Save
0 /5.0
User reviewRetrospective Study Design and Data Analysis for Clinical, Epidemiological, and Real-World Evidence Research
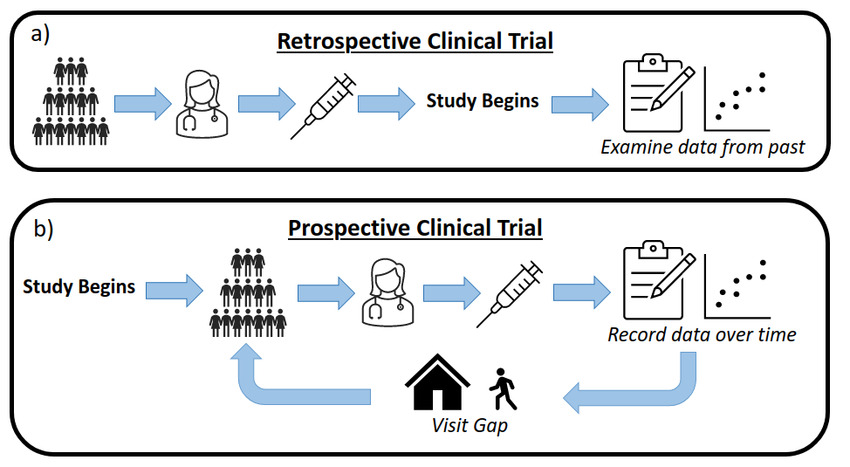
Are you conducting a retrospective study using hospital records, registries, or electronic health data? This service provides full statistical support for the design, analysis, and reporting of retrospective cohort and case-control studies aligned with international publication standards.
Whether your aim is to identify risk factors, compare treatment outcomes, or estimate survival probabilities, I deliver robust, reproducible analyses suitable for peer-reviewed journals and regulatory submissions.
What’s Included
- Study design consultation (retrospective cohort or case-control structure)
- Data cleaning, variable coding, and handling of missing or incomplete records
- Descriptive and comparative statistics for baseline characteristics
- Multivariable logistic and linear regression models
- Time-to-event (survival) and competing-risk analyses
- Propensity score matching (PSM), weighting, or adjustment
- Sensitivity and subgroup analyses
- Effect size estimation with 95% confidence intervals
- Publication-ready Tables, Figures, and Listings (TFLs) formatted in APA or AMA style
- Syntax/code (SPSS, R, or Stata) for full reproducibility
Expertise
- Software: R, SPSS, SAS, and Stata
- Study Types: Hospital record reviews, registry-based analyses, electronic health record (EHR) studies, case–control, and matched cohort designs
- Publication Standard: APA 7th / AMA / CONSORT / STROBE compliance
- Confidentiality: All data handled securely under NDA upon request
Fequently asked questions
1. What types of cohort studies do you support?
I work with both prospective and retrospective cohort studies, including registry-based, hospital-based, and population-based datasets, as well as real-world evidence studies.
2. How do you ensure patient data privacy?
All data are handled under strict confidentiality, stored securely, and never shared with third parties. I can sign an NDA or data use agreement if required.
3. Which statistical methods are typically used for cohort analysis?
I apply logistic regression, Poisson regression, Cox proportional hazards, Fine–Gray competing risks, and longitudinal mixed models depending on your study design and outcomes.
4. Can you analyse time-to-event outcomes in cohort studies?
Yes, I conduct survival analysis using Kaplan–Meier, Cox models, and competing risk methods for outcomes such as mortality, disease progression, or treatment response.
5. How do you deal with missing data in longitudinal cohorts?
I use techniques such as multiple imputation, mixed-effects modelling, and sensitivity analyses, always aligned with ICH E9(R1) recommendations.
6. Do you provide publication-ready results?
Yes, I deliver outputs as structured tables, figures, and listings (TFLs) suitable for journal submission or reports, along with clear explanations of statistical methods.
7. Which software do you use for cohort analysis?
I primarily use R, SAS, and Stata for advanced modelling. SPSS can be used for applied healthcare datasets if requested.
8. Can you identify risk factors and associations?
Yes, I perform univariate and multivariable regression analyses to identify predictors, associations, and potential confounders, adjusted for relevant covariates.
9. What information do I need to provide to start?
A dataset or access to your registry or list of variables, a clear research question, defined outcomes, and any covariates of interest are sufficient to begin the analysis.
10. Do you also help with writing methods and results?
Yes, I can prepare the statistical methods and results sections for manuscripts or reports, ensuring clarity, accuracy, and compliance with publication standards.